It works for the same fundamental reason as any form of ionizing radiation: it
causes changes in the atoms of diseased cells that lead to changes in those
cells and cause them to die or stop functioning. It offers an excellent means of
noninvasively treating patients with localized cancers, and, owing to fewer side
effects, because patients usually experience better quality of life after proton/ion
treatments than after other forms of radiation. The twin goals of controlling
disease and minimizing side effects are the classic aims of radiation treatment;
hadrontherapy enhance the opportunity for both.
The main advantage of the use of protons and ions in radiotherapy relies in
their dose distribution curves that they releases traversing tissues.
The depth-dose curves for
proton beams are completely different from those of convential radiation beam
used in radiotherapy: i.e. photons and electrons. Charged particles have little
scattering when penetrating in matter and give the highest dose near the end of
their range just before coming to rest. This is the well known "Bragg
Peak".These characteristics permit one to control very precisely the shape
of the dose distribution inside the patient's body.
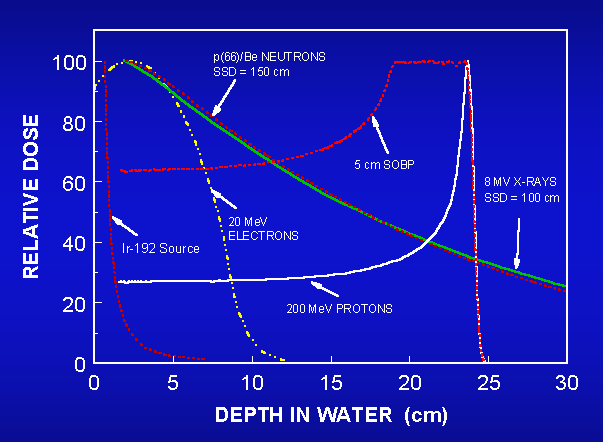
Figure 1 presents the comparison of the percentage of the absorbed dose (in
water) for different radiotherapic sources: a brachytherapy 192-Ir gamma source,
20 MeV electrons, 8 MV X-Ray beam, p(66)/Be neutrons, monochromatic 200 MeV
proton beams (Bragg curve), an a "modulated" proton beam, the so-calle
Spread Out Bragg Peak (SOBP).

Figure 1. Dose distribution curves for different
radiation source used for the raditherapic treatment of tumours.
It is evdent as protons deposit their radiation dose differently than x-ray or
electron beams do. Compared to an x-ray beam, a proton beam that is delivered
with sufficient energy (or "modulated") has a low "entrance
dose" (the dose in front of the tumor), a high-dose "Bragg peak"
region, which is designed to cover the entire tumor, and no "exit
dose" beyond the tumor. X-ray beams may deposit most of their dose in
tissues in front of the tumor.
Today
more than 25 centres, using proton and carbon ions for radiotherapeutic purposes,
are active [1]. Beside of this, a huge number of studies are yet necessary to
improve the quality of an ion therapy treatment. These studies regard both a
better understanding of the dose distributions inside patients (also taking into
account the contribute due to the nuclear non-elastic interactions) as well as
the possibility to improve the design of the transport beam line dedicated to
the ion therapeutic treatments.
On the basis of the experience we gained in the last years in the realization of the CATANA facility [2, 3], dedicated to the treatment of ocular melanoma using 62 MeV proton beams, and considering the future proton therapy facilities that will be developed in the world [1], in the next years, we decided to start the development of a Monte Carlo application dedicated to proton/ion therapy and based on the Geant4 toolkit [4].
References
[1] J. Sisterson, Links on
ion-therapy facilities, Particles Newsletters, http://ptcog.mgh.harvard.edu.
[2] L. Raffaele, G. A. P. Cirrone, G. Cuttone, S. Lo Nigro, M. G. Sabini, V.
Salamone, E. Egger, A. Kacperek, N. Romeo,
Proton beam dosimetry for the CATANA project,
Physica Medica XVII, Supplement 3, 35 – 40 (2001).
[3]
G.
A. P. Cirrone, G. Cuttone, P. A. Lojacono, S. Lo Nigro, V. Monelli, I. V. Patti,
G. Privitera, L. Raffaele,
D. Rifuggiato, M. G. Sabini, V.Salamone, C. Spatola, L. M. Valastro,
A 62 MeV proton beam for the treatment of ocular melanoma at Laboratori
Nazionali del Sud - INFN,
Transaction on Nuclear Science, in press.
[4]
S.Agostinelli,
J. Allison, K. Amako et al.,
Geant4-simulation
toolkit, Nucl. Instrum. Method. Sec. A, 506 Issue 3, 250-303 (2003).